Massively Scalable Neurotechnologies: A possible ARIA programme
Dive into this guest blog from Programme Director, Jacques Carolan, on his thinking around a potential new programme.
What if treating severe depression was as simple as a flu jab? A single injection that precisely and actively tunes your brain's mood circuits for months at a time.
What if we could reverse Parkinson's with a nasal spray? A cell therapy that autonomously replaces lost neurons, dramatically improving motor function without brain surgery.
Or if a simple blood test could give your psychiatrist a biological report from your brain? A direct readout to diagnose your condition and select the right treatment on the first try.
Powerful treatments for devastating brain disorders are on the horizon. The problem? They are fundamentally unscalable, accessible only to a tiny fraction of the millions who need them. This isn't a gap; it's a failure of imagination. I'm scoping a new ARIA programme to address this.
The context:
We’re experiencing a global brain-health crisis: brain disorders are the single biggest disease burden globally, twice that of cardiovascular disease and stroke, with annual costs in Europe and the USA estimated to be $1.7 trillion for neurological conditions alone.
Targeted interaction with the human brain can significantly improve outcomes: whether that’s deep brain stimulation (DBS) for intractable neuropsychiatric disorders or cell therapies for neurodegenerative disease.
Current interventions will not meet the overwhelming need: one of our most well-tested and reimbursed implanted neurotechnologies, DBS for Parkinson’s disease, is not increasing at a sufficient rate to meet demand.
There is a confluence of advances in molecular and cellular biology, nanotechnology and applied physics that now make a truly scalable platform possible — designed from the outset to reach millions of people and increase access globally.
Neurotechnologies scalability crisis
DBS stands as a landmark neurotechnology. FDA-approved for Parkinson's disease for over two decades, it is an existence proof that targeted electrical stimulation can be a powerful treatment for brain disorders. Its true significance, however, may lie in its growing promise for a much broader range of intractable neuropsychiatric conditions.
This makes its failure to scale all the more consequential. The number of people who could potentially benefit from this class of therapy is increasing many times faster than the number of procedures performed annually. This is not a gap; it is a systems-level failure of profound consequence.
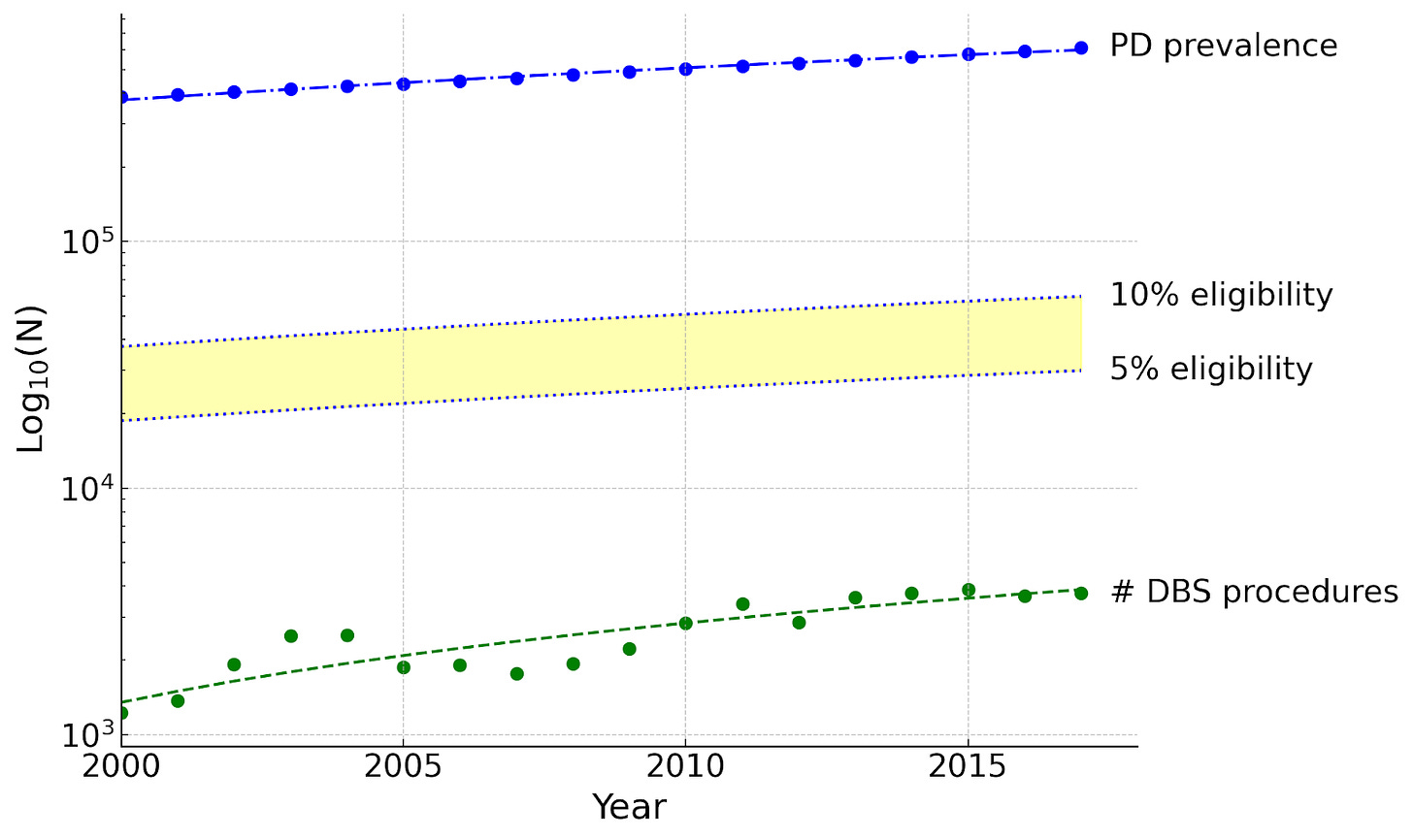
This failure is the central challenge in neurotechnology. The problem is not a lack of efficacy, but a lack of a viable path to scale. The requirement for complex surgical procedures imposes a series of stacked, multiplicative barriers that fundamentally limit the scalability of any therapy, regardless of its potential.
Let’s break these factors down: first, the number of clinicians who can perform these procedures is limited and increasing only modestly. Solving the human capital challenge has a decadal time constant. Second, the number of locations where these procedures can be performed is also limited and the infrastructure is expensive to build, limiting access to a handful of urban medical centres, creating geographic and economic inequities where treatment is simply unavailable. Finally, breaching the dura mater and implanting a medical device carries a non-negligible risk of infection. This gives rise to patient hesitancy and, at a population level, would place a further burden on already stretched healthcare systems.
These barriers limit emerging therapies to only the most severe, treatment-refractory patients, excluding the vast majority who could benefit from earlier, less risky intervention. Together, they lock neurotechnology into a low-volume, high-cost, last-resort paradigm. To escape this trap, we need a paradigm shift — a 1000x leap in what is possible.
Case study: cardiology in the 1950s
To understand what a 1000x increase in scalability looks like, we need only look to the history of cardiology. In the 1950s, cardiology faced a challenge analogous to that facing neurotechnologies: treating heart block required open-heart surgery. The field didn’t scale by improving that surgery; it scaled by making it obsolete with the transvenous lead. This innovation turned a high-risk operation into a routine procedure now performed millions of times per year.
This offers three critical lessons:
Solving invasiveness drives scale: The leap from open-chest surgery to a simple catheter procedure was the catalyst that unlocked exponential growth, turning a niche device into a global standard of care.
A foundational platform creates a new market: Despite initial technical issues, the core platform of a minimally invasive, implantable device created the conditions for a new industrial ecosystem to form. This market then drove rapid, iterative innovation to solve secondary problems such as battery life and reliability.
New indications emerge from a reliable platform: Initially used for a single condition, the platform’s improving safety and reliability led clinicians to discover and validate a host of new applications. A safe, scalable platform technology will unlock therapeutic possibilities far beyond its initial use case.
The transvenous lead did not merely improve the existing system; it created a new one. It unlocked a new class of clinician, a new clinical setting, and a new risk–reward calculation for patients, creating the conditions for a massive industrial ecosystem to form and drive rapid innovation. This is the nature of a true leap in scale.
Emerging signals
The pacemaker revolution of the 1950s and ‘60s was enabled by the concurrent rise of the microelectronics industry. This historical parallel begs the question: what technological tailwinds will enable the vision of ‘surgery-free’ neurotechnologies? We believe the answer lies at the intersection of engineered biology and engineered hardware.
This convergence unlocks a new design space for bi-directional neural interfaces, creating unprecedented capabilities in three key areas:
Autonomous delivery: We can now design biological vectors, like engineered cells or viruses, that can be systemically administered to cross the blood-brain barrier and autonomously navigate to deep brain targets. On a more macroscopic scale, we can envision micro- or milli-implants autonomously traversing the vasculature or cerebrospinal fluid to reach their destination without transcranial surgery.
Wireless therapeutic action: These delivery vectors can carry novel payloads, such as bioelectronic transducers, or sonogenetic or magnetogenetic actuators for the wireless control of neural circuits. They could even carry the tools for regenerative medicine, replacing neurons lost to disease by delivering new cells or reprogramming existing ones in vivo.
Non-invasive readout: These same systems can act as biological reporters, engineered to monitor brain health and send disease-relevant biomarkers out of the brain. This could enable a ‘liquid biopsy for the brain’, where vital information is read out via a simple blood test or other non-invasive signals.
Targets for a new ARIA programme
So having reflected on these lessons and emerging signals, here’s how we’re thinking about the ‘north star’ of a programme to drive scale:
Validated therapeutic efficacy: To overcome the immense inertia of the current system, it must demonstrate a functional impact at least equivalent to, and ideally superior to, today's best-in-class interventions. That means accessing clinically validated brain targets.
Non-surgical access: The therapy should be delivered without breaching the dura mater transcranially. Can we leverage systemic routes, such as intravenous injection? Or the cerebrospinal fluid via a lumbar puncture, or the vasculature via catheterisation? Or even utilising direct neural pathways — such as intranasal delivery?
Radical simplicity: The procedure must be simple enough to be performed by a non-specialist with minimal training, in under 30 minutes, in a standard outpatient clinic or catheterisation lab. This is the only way to break the bottleneck of specialist clinicians and centralised hospital infrastructure.
We know we don't have all the answers, and we’re looking for the people and ideas that will challenge our thinking. If you are working on transformative, unconventional solutions that could lead to a 1000x shift in scale, we want to hear from you. Here are some questions on our minds:
Non-invasive: How far can purely non-invasive routes take us (e.g., transcranial ultrasound) in terms of targeting accuracy for deep brain structures? Can this be achieved in a portable, small-volume form factor acceptable to users (like a behind-the-ear hearing aid or earbud) or will you always need large-scale ‘helmet scale’ equipment? How do micro- or nano-scale transducers change this calculus?
Metrics: What is the single procedural metric (e.g. procedure time, training hours, sterility class, reversibility) that, if radically improved, would have the greatest non-linear impact on scalability?
Access routes: What are some of the most overlooked anatomical pathways to the brain (e.g. lymphatic system, bone marrow channels, the cribriform plate)?
Readout: What biomarkers would be valuable for assessing neurological and neuropsychiatric disorders (e.g. LFP, neurotransmitter concentration, gene expression) and from which specific brain regions?
Vector delivery: What new delivery vectors can provide blood–brain barrier crossing and brain-region-specific targeting? How do you minimise off target effects such as liver accumulation?
Power delivery: Are there ways to harness local energy sources such that we could obviate the need for continuous, external power delivery?
Targets: What are the most impactful deep brain targets (e.g. STN, SCC, fornix) and what is the necessary spatial targeting accuracy?
Autonomy: What role might robotics play in highly scalable, autonomous neuro-implantation? Will this always require a million-pound machine or can it be done with low-cost or even existing accessible hardware?
Imaging: Are there novel and miniaturised imaging modalities that we can use to precisely localise implants as they traverse the body, without having to rely on large-scale radiology equipment?
Materials: What advances in materials science will unlock new capabilities for neural interfaces? Can we envision next-generation endovascular devices that don’t require patients to take blood thinners? Or neurostimulators that biodegrade on command?
Massive thanks to Aayush Chadha, Gillian Koehl, Edward King and Alicia Cooke for help in preparing this article.

This was an essential direction within the US NIH Brain Initiative. The results of the research essentially got commercialised into Elon Musk's Neuralink company. But that does not mean we should not do this!
It costs around $250-500 million to bring a medical device company to market. As such, up until recently, the only direction for commercialisation was essentially to create a company and eventually sell to a large US corporation like Abbot or Medtronic. However, existing MedTechs are primarily focused on pacemaker-like systems, whether for the brain or the heart. A multisite cortical or deep-brain implant is quite far from their regulatory and manufacturing expertise.
As such, while I think the UK should push for its own indigenous companies, at very least with Neuralink and more recently perhaps a new Sam Altman rival, there will be a commercialisation pathway.
Furthermore, with proper funding, there are opportunities to really rethink the current brain interface. So let's do this! :-)
Really exciting to see ARIA leaning into this space and trust Jacques to set out an ambitious proposal! Here are my two cents.
I think from a mental health perspective there are some challenges that are quite distinct from other brain disorders. Unlike Parkinson’s or epilepsy, where there are relatively well-defined neural targets, mental health conditions are heterogeneous and do not map neatly into single circuits, so precision will be a lot harder to deliver. There is also the perennial translation gap. Showing that modulating neural activity can produce meaningful and sustained improvements in mood or function (notoriously difficult to even define and measure), beyond placebo, will be a major hurdle. Add to the mix issues of safety and tolerability in a field where trust is fragile. That said, I believe that if these challenges are confronted head-on, scalable neurotechnologies could open an entirely new frontier for mental health, and ARIA is uniquely placed to drive a bold and transformative initiatives in this space. Best of luck!